Please note that this is a draft schedule and will inevitably change to some extent.
1 | 8/28 | Starter Cultures

In Class
- Welcome
- Lecture: What is Biofabrication?
- Website Overview
- Syllabus
Video: Suzanne Lee- Introduction to the Bio Lab
- EHS
- Communication
- Lab Work: G.hansenii Starter Culture for growing Bacterial Cellulose
- Work in teams of two students
- Make HS Media
- Each team will make 1L of media
- Inoculate 10 mL cultures
- 2 cultures per student
- one negative control per team
Homework
- Website
- if your Google profile picture is not a recognizable image of yourself, please change it
- log in to the class website, using the “continue with google” option
- confirm that your photo shows up on the the class People page
- if needed, you can make changes to your “display name” on your profile page
- Lab
- finish making media (if needed)
- check on cultures for cellulose growth -> pellicle formation -> thickness of 2mm+
- check on Negative Control
- Reading
- Prepare for next week’s lab session:
- Review units of measurement for the bio lab
- Watch pipetting tutorial videos (~7 minute)
2 | 9/04 | Labor Day
3 | 9/11 | Culture Growth
In Class
- Discussion
- Presentation
- Video – Suzanne Lee: Grow your own clothes
- Lecture – Biofabrication with Bacterial Cellulose
- Lab
- scale up G. hansenii cultures to 200mL in 500mL Erlenmeyer flasks
- discuss next scale up to 1-5L
- look at cells and cellulose under the microscope
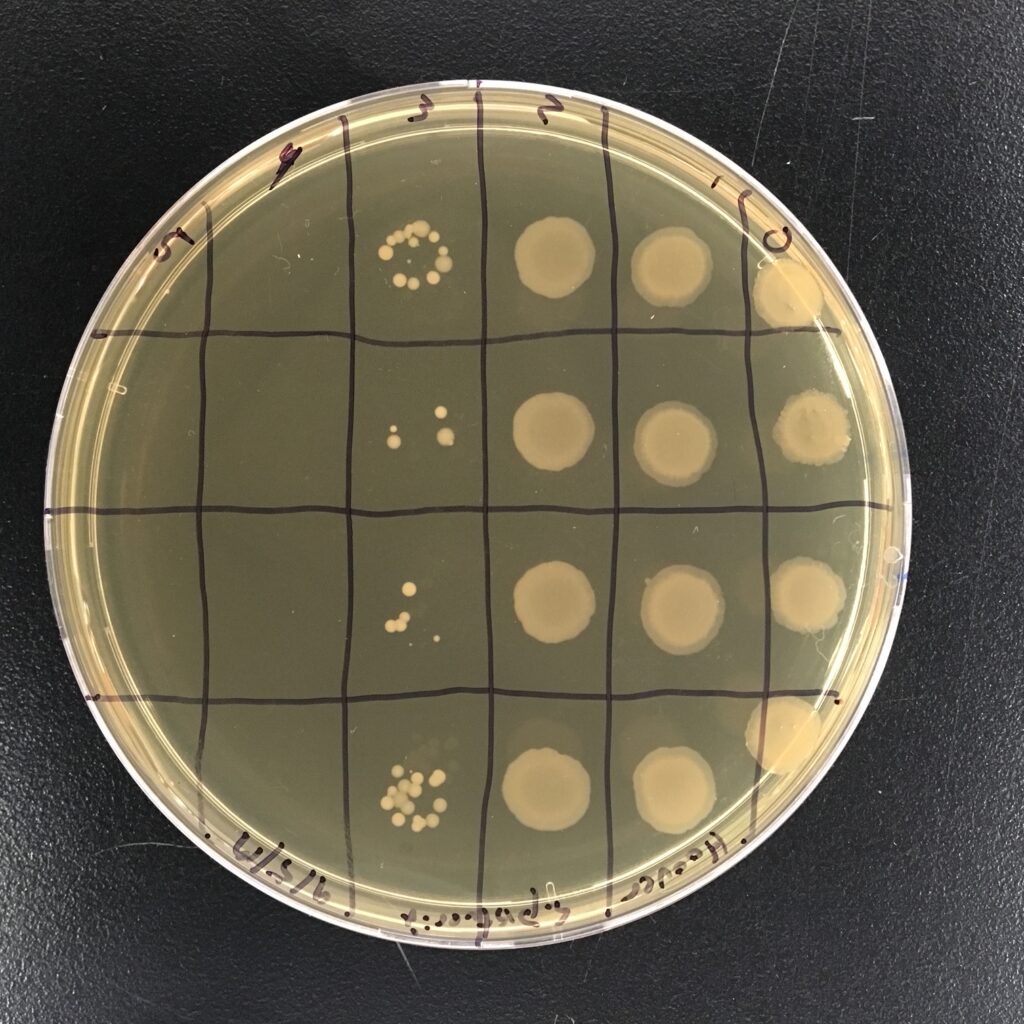
- serial dilution lab exercise to determine cell culture density
Homework
- Lab
- Serial Dilution
- check on plates
if you cannot make these times, ask a classmate to photograph your plates
once you have readable plates, no further check-in are required
additional dates will be scheduled if needed- Tues 3:00-4:00
- Wed 2:30-3:30
- once plates are readable
- count the colonies on the plates
- photograph the plates
- log the data in the spreadsheet
- check on plates
- G. hansenii Cultures
- begin planning your bacterial cellulose project
- make HS media
- determine volume needed, based on the desired size pellicle and needed vessel
- make media and autoclave it
- consolidate your media as soon as possible to free up bottles
- Serial Dilution
- Reading
- Prepare for next week’s lab session:
- Review units of measurement for the bio lab
- Watch pipetting tutorial videos (~7 minute)
4 | 9/18 | Scaling Up

In Class
- Lab
- examine serial dilution exercise results
- review plates
- spreadsheet data
- discuss results
- examine 200mL G. hansenii cultures
- scale up G. hansenii cultures to 1-5L
- look at G. hansenii and cellulose under the microscope
- examine serial dilution exercise results
- Lecture
- Discussion
- Synthetic Biology: A Primer, Ch 1
Homework
- Read (printouts are in the cubbies just outside of the lab)
- CRISPR lab guide pgs 1-21
- Do worksheet exercises in Part I
- Synthetic Aesthetics Ch1 “Synthetic Biology: What It Is and Why It Matters” by Alistair Elfick and Drew Endy
5 | 9/25 | CRISPR Part I – Classroom
In Class
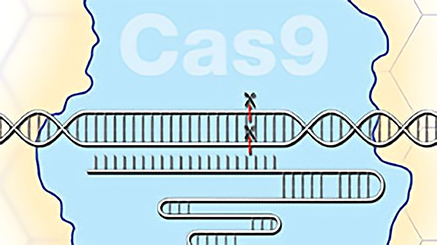
- Lecture: Genetic Engineering with CRISPR
- Review CRISPR text and worksheet
- Prepare for CRISPR lab
- Discuss Synthetic Aesthetics Ch1 “Synthetic Biology: What It Is and Why It Matters” by Alistair Elfick and Drew Endy
- Introduce 2D Bioprinting Project
- Lecture: Artists using synthetic biology
- check on G. hansenii cultures
Homework
- review CRISPR Lab
- begin planning 2D Bioprinting Project
6 | 10/02 | Class Canceled
Homework
- review CRISPR Lab
- CRISPR lab guide pgs 10-21
- Slide lecture (slides 35-57)
- begin planning 2D Bioprinting Project
7 | 10/09 | CRISPR Part II – Lab

In Class
- CRISPR lab
- check on G. hansenii cultures
- 2D Bioprinting
- Presentation: example work
- Techniques
- direct drawing with various tools
- stamps
- stencils
- hand cut
- laser cut
- image to stencil Grasshopper template
- bioprinter
Homework
- check on CRISPR plates
- Tues 3:00-4:00
- Wed 2:30-3:30
- begin planning and preparing for 2D Bioprinting Project
- gather tools
- create stencils
- prepare files
- pour agar plates
8 | 10/16 | Fall Break
9 | 10/23 | Bioprinting Work
10 | 10/30 | Critique Bioprinting Project – Cellulose Harvest

In Class
- Critique
- Harvest Bacterial Cellulose and begin washing protocol
Homework
- complete washing protocol
- This process involves daily steps. Share the labor with classmates as needed to make this manageable
- Tuesday 3:15-4:15
- Wednesday 3:15-4:15
- Thursday 2:45-3:45
- Friday 12:00-1:00
- Saturday 12:00-1:00
- Sunday 9:00-12:00 (limited support) 12:00-12:45 (full support)
- This process involves daily steps. Share the labor with classmates as needed to make this manageable
11 | 11/06 | Cellulose Conditioning
In Class
- demo on cellulose treatment methods
- work time
Homework
- complete cellulose treatment samples
- work on treatment of final cellulose pellicles
- Lab Hours
- Wednesday 4:00-6:30
- Friday 9:00-4:00 (limited support)
12 | 11/13 | Mycelium Inoculation

In Class
- check in on cellulose project
- one student crit of cellulose project
- walkout 10-11:30
- prepare substrates for mycelium project
Homework
- autoclave mycelium substrates – [completed by Ryan]
- innoculate substrates with mushroom cultures
- complete bacterial cellulose project
- Lab hours:
- Tues 9:30-4:00
- Wed 8:30-2:30 (unsupported), 2:30-5:00 (supported)
- Thursday 2:00-3:30
- Friday 9:00-11:00 (supported), 11:00-4:00 (unsupported)
13 | 11/20 | Critique Bacterial Cellulose Project

In Class
- 9:00-9:30 Install
- 9:30 Critique
- check on mycelium cultures
vacuum forming demo
Homework
- prepare a mold for mycelium casting -or- bring a pattern ready for vacuum forming
- lab cleanup
- document bacterial cellulose project
- post project documentation
14 | 11/27 | MycoCasting

In Class
- presentation: mycelium in art, design, and manufacturing
- lab clean up
- vacuum forming demo
- fill molds with inoculated substrate
Homework
- fill molds with inoculated substrate (if not done in class)
- document process
- reading: A Symbiotic View of Life: We Have Never Been Individual – paper by Scott Gilbert, et. al.
- related recommended readings:
- I Contain Multitudes – book by Ed Yong – Chapter 1 Living Islands
- Ways of Being – book by James Bridle – Chapter 1 Thinking Otherwise, Chapter 2 Wood Wide Web
- Staying with the Trouble – book by Donna Haraway
- related recommended readings:
- Lab hours:
- Tues: 9:00-5:00 – unsupported
- Wed: 8:30-7:00 – unsupported
- Thurs: 9:00-4:00 – unsupported
4:00-6:30 – supported - Fri: 9:00-4:00 – unsupported