Please note that this is a draft schedule and will inevitably change to some extent.
weeks 1-7 – genetic engineering and bioprinting
weeks 9-16 – growing and working with bacterial nanocellulose
weeks 13-16 – growing and working with mycelium
1 | 8/30 | An Introduction to Biofabrication

In Class
- Welcome
- An Introduction to Biofabrication
- course overview
- syllabus
- website
- ethics
- Lab Overview
- lab safety
- lab policies
- tools of the biology lab
- making media (time permitting)
- presentations and discussions
Homework
- READ: Synthetic Aesthetics Ch 1, Drew Endy and Alistair Alfick , and Donna Haraway’s Anthropocene, Capitalocene, Plantationocene, Chthulucene: Making Kin
- PLAN: Prepare for next week’s lab session:
- Review units of measurement for the bio lab
- Watch pipetting tutorial videos (~7 minute)
2 | 9/6 | Intro to Synthetic Biology
In Class
- Discuss readings
- Lab I – cell culture density analysis
- Lab safety
- optical density
- serial dilutions
- Pipetting
- Positive and Negative control
- lab notes
- set times for lab access
- overview of synthetic biology
- DNA structure
- genetic parts
- engineered standardized parts
- restriction enzymes
- biobricks assembly
- CRISPR
- Introduce Lab II
Homework
- complete Lab I
- counts cells according to the protocol
- log data in spreadsheet
- Review the protocol for next week’s lab work
- read pgs 1-3
- skim 3-21
- READ:
3 | 9/13 | Lab II – Genetic Engineering with CRISPR+Cas9

In Class
- Review results of Lab I
- Review readings
- Technical overview of genetic engineering
- with restriction enzymes
- with CRISPR+Cas9
- Lab II – CRISPR+Cas9
- paper model (moved to homework)
- lab exercise
Homework
- complete questions in lab protocol handout (“Activity 2”)
- complete paper CRISPR worksheet
- gather material and ideas for 2D Bioprinting experimentation
4 | 9/20 | 2D Bioprinting Experimentation

In Class
- review CRISPR lab results and discuss
- explore different bacterial printing and drawing processes
- Lecture: bacterial printing examples
Homework
- review results of experiments
- plan for project I
- prepare materials, tools, and/or files for in-class work
5 | 9/27 | 2D Bioprinting Experimentation
In Class
- Lecture: BioArt Gen I
- Print with genetically modified bacteria
- Discuss bioprinting project ideas
Homework
- DO: complete bacterial prints
- document process
- photograph results
6 | 10/4 | Bioprinting Work Day
In Class
- work
- document
Homework
- Complete Bioprinting Project
- document bacterial prints
7 | 10/11 | Critique
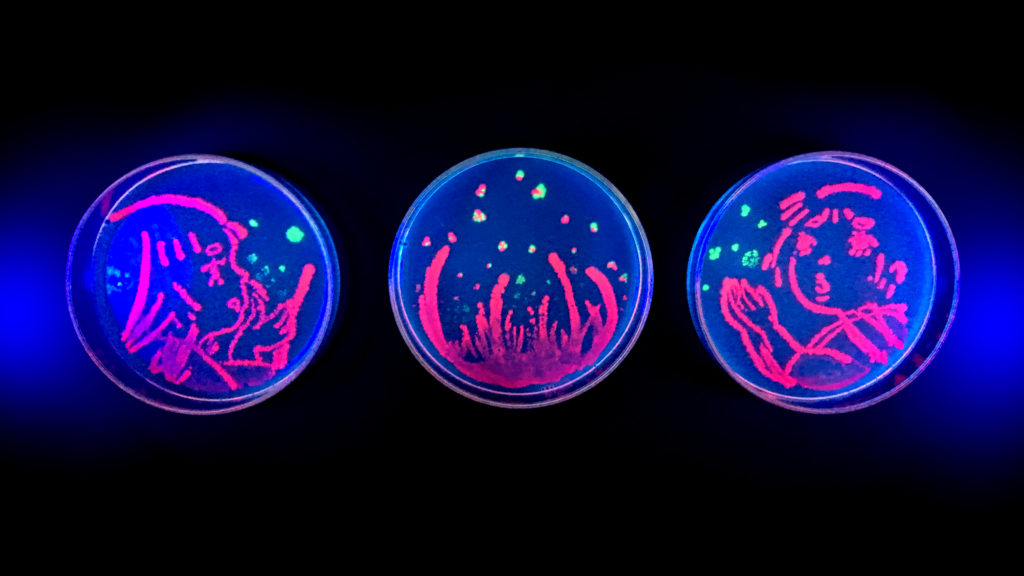
In Class
- Critique
- Lab cleanup
Homework
- document
- create post on class website documenting your bioprinting project and process
8 | 10/18 | Fall Break
9 | 10/25 | Bacterial Cellulose

In Class
- Reading Discussion: Resisting Biopolitics, Introduction
- Lecture: Bacterial Cellulose
- Lab: Bacterial Cellulose
- make media
- sterilize tubs
- inoculate media
- place tubs in incubator
Homework
- Agitate tubs
- Resisting Biopolitics assignment
- select an essay from Resisting Biopolitics to read
- prepare to give a 5-10 minute summary in class
10 | 11/1 | Bacterial Cellulose Growth
In Class
- Complete bacterial cellulose setups
- Connect air lines to tubs
- Review how to post your work to this site
Homework
- Resisting Biopolitics assignment
- read selected essay from Resisting Biopolitics
- prepare to give a 5-10 minute summary in class
- monitor bacterial cellulose cultures for contamination
- log all observations
- research bacterial cellulose applications and material treatments
- create post of bioprinting projects
11 | 11/8 | Biopolitics
In Class
- discuss Biopolitics essays
- discuss biomaterials project proposals
Homework
- research bacterial cellulose applications and material treatments
12 | 11/15 | Bacterial Cellulose Work Day
In Class
- harvest bacterial cellulose
- begin bacterial cellulose washing protocol
- begin material experiments
Homework
- read I Contain Multitudes, Ch 1 (printouts are in the cubbies, outside the lab)
- continue bacterial cellulose washing protocol
- continue material experiments
- document experiments with photos and data
- collect mycelium substrates
- common substrates are straw, grain, sawdust (hardwood is often preferred), and other cellulosic starchy materials
- choose 3 materials for experimentation
- have enough of each material to fill 4 regular petri dishes
- choose at least 1 material for your project
- this should be one of your three experimental materials
- make sure you have ample material to fill your mold
- molds can still be TBD for now, but plan for something roughly 0.5L – 3L in volume
- autoclave substrates (if available Monday 3:00-4:00)
13 | 11/22 | Mycelium Biomaterials
In Class
- autoclave remaining mycelium substrates
discuss I Contain Multitudes- overview of mycelium-based biomaterials
- inoculate substrate
- bulk material
- petri dish samples
vacuum form demowatch Fantastic Fungi (time permitting)- continue work on bacterial cellulose processing
Homework
- continue work on bacterial cellulose processing
- create/find mold for mycelium project
- give thanks
14 | 11/29 | Mycelium Forming
In Class
- inoculate autoclaved substrate materials with mycelium cultures
- create petri dish samples
- vacuum form demo
- pack inoculated and grown materials into molds
- continue work on bacterial cellulose
- discuss I Contain Multitudes
- watch Fantastic Fungi
Homework
- complete bacterial cellulose project
- document bacterial cellulose project
- monitor mycelium
- if mycelium is ready pop it out of the mold and tent it for final surface growth
15 | 12/6 | Final Work Day
In Class
- check on petri dish samples
- if primary growth is complete, remove it from the petri dish and tent it for final surface growth
- if all growth is complete, dry it in toaster oven
- check on mycelium castings
- if primary growth is complete, pop it out of the mold and tent it for final surface growth
- if all growth is complete, dry it in toaster oven
Homework
- complete mycelium project
- document mycelium project
- create a post on the class website for your bacterial cellulose project
- create a post on the class website for your mycelium project
16 | 12/13 | Final Critique of Cellulose and Mycelium Projects
In Class
- critique


