Please note that this is a draft schedule and will inevitably change to some extent.
1 | 8/27 | Starter Cultures

In Class
- Welcome
- Website Overview
- Syllabus Review
- Lab Hours
- Lab Notebooks
- Introduction to the Bio Lab
- EHS
- Communication
- Lab Demo: Making Growth Media
- Lab Work: K.hansenii Starter Culture for growing Bacterial Cellulose
- Inoculate 10 mL cultures with K. hansenii cell from a -80C storage culture
- 2 cultures per student
- 1 negative control
- place in the static incubator until next class
- Inoculate 10 mL cultures with K. hansenii cell from a -80C storage culture
- Find a lab partner for homework
Homework
- Website
- if your Google profile picture is not a recognizable image of yourself, please change it
- log in to the class website, using the “continue with google” option
- confirm that your photo shows up on the the class People page
(log out and back in from the class website to see changes made via your google account) - if needed, you can make changes to your “display name” on your profile page
- Lab
- check on cultures for cellulose growth -> pellicle formation -> thickness of 2mm+
- check on Negative Control
- Reading
- Prepare for next week’s lab session:
- Review the Serial Dilution Protocol
- Review units of measurement for the bio lab
- Watch pipetting tutorial videos (~7 minute)
- Submit your general availability to work in the lab via this schedule survey.
2 | 9/03 | Culture Growth
In Class
- Discussion
- Presentation
- Lecture: An Introduction to Biofabrication
- Video: Suzanne Lee “Why “biofabrication” is the next industrial revolution”
- Lab
- examine 10mL K. Hansenii cultures
- check to see if there is a pellicle
- note the thickness of the pellicle
- snap a photo of your culture, focused on the pellicle at the top
- scale up K. hansenii cultures
- Add ~ 200mL of HS media to a sterile 500mL Erlenmeyer flasks, and repeat for a second flask.
- Add all 10mL of the culture and the pellicle from each test tube to each Erlenmeyer flask
- Secure the sponge closure on the Erlenmeyer flask
- Place the flask in the shaker incubator set to 28C
- shake overnight then move to static incubator until next week
- discuss next scale up to 1-5L
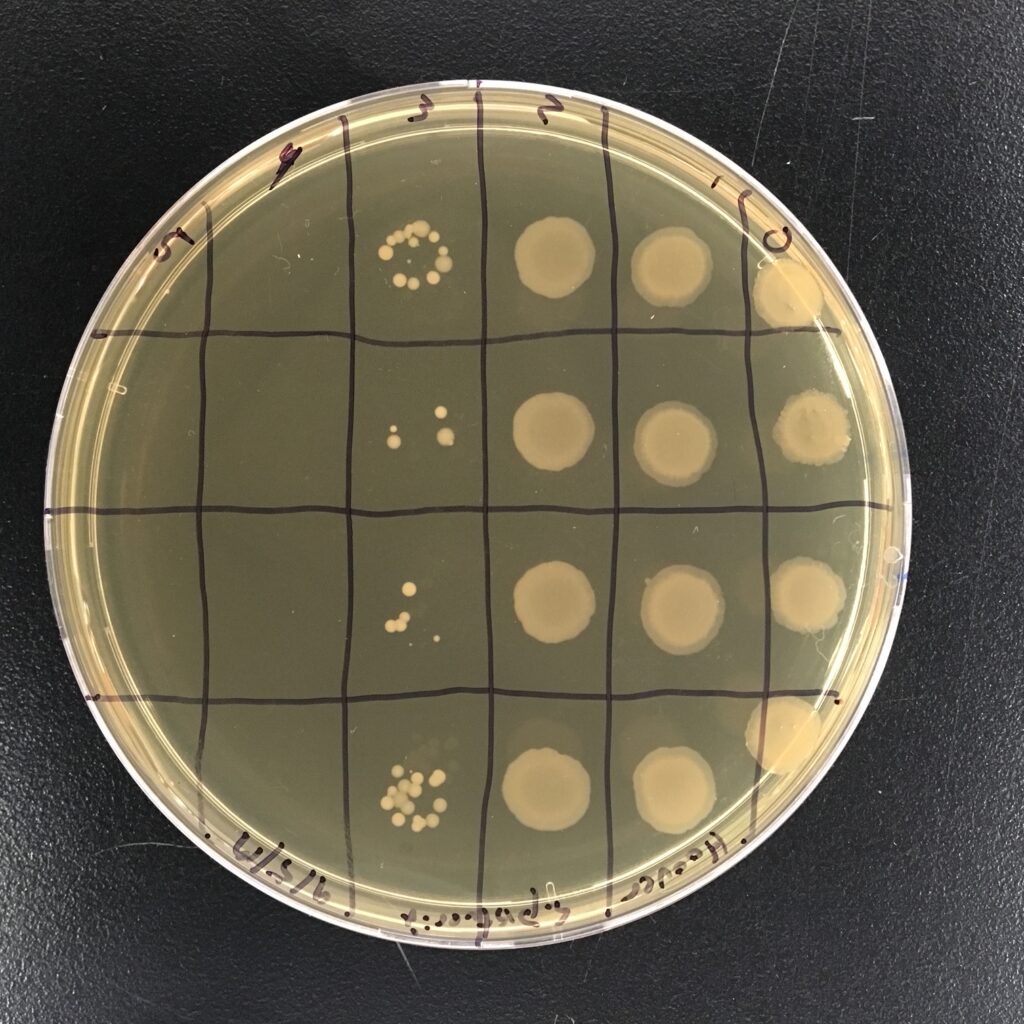
- serial dilution lab exercise to determine cell culture density
- observe cells and cellulose under the microscope
- examine 10mL K. Hansenii cultures
Homework
- If you have not done so already, submit your general availability to work in the lab via this schedule survey.
- Lab
- Serial Dilution
- check on plates the next day during scheduled lab hours
if you cannot make these times, ask a classmate to photograph your plates
once you have readable plates, no further check-in are required
additional dates will be scheduled if needed- see lab calendar for times
- once plates are readable
- count the colonies on the plates
- photograph the plates
- log the data in the spreadsheet
- check on plates the next day during scheduled lab hours
- K. hansenii Cultures
- begin planning your bacterial cellulose project
- make HS media
- determine volume needed, based on the desired size pellicle and needed vessel
- make media and autoclave it
- consolidate your media as soon as possible to free up bottles
- Serial Dilution
- Reading
- Prepare for next week’s lab session:
- Review units of measurement for the bio lab
- Review pipetting tutorial videos as needed
3 | 9/10 | Scaling Up

In Class
- Lecture: An Introduction to Biofabrication
- Discussion: Donna Haraway’s Anthropocene, Capitalocene, Plantationocene, Chthulucene: Making Kin
- Lab: serial dilution exercise
- review plates
- spreadsheet data
- discuss results
- Lecture: Molecular Biology for Genetic Engineering
- Discussion: Synthetic Biology: A Primer, Ch 1
- Lab: K. hansenii cultures
- examine 200mL K. hansenii cultures
- discuss scale up K. hansenii cultures to 1-5L
- look at K. hansenii and cellulose under the microscope
- Video: Suzanne Lee “Why biofabrication is the next industrial revolution”
- Lecture
Homework
- Lab
- Scale up K. hansenii cultures to 1-5L
- Read
4 | 9/17 | Synthetic Biology Part I – Classroom
In Class
- Lecture:
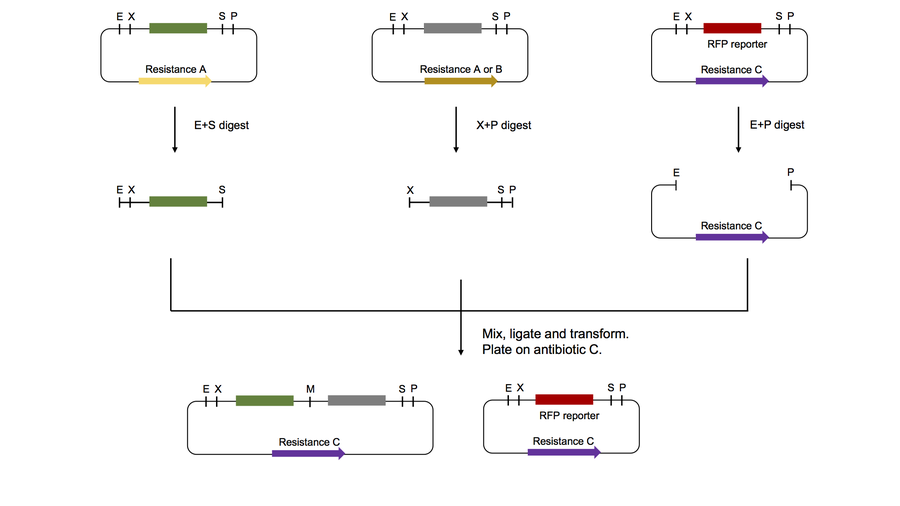
- BioBricks Assembly
- Protocol for Synthetic Biology Lab
- Review Synthetic Biology reading
- Introduce 2D Bioprinting Project
- Lecture: catch up from last week
- Lecture: Artists using synthetic biology (time permitting)
- check on K. hansenii cultures
Homework
- check on K. hansenii cultures
- Videos: BioBricks Assembly with Jake Wintermute
- review Synthetic Biology: A Primer
- review pipetting videos (accurate pipetting with very small volumes will be critical for lab)
- begin planning 2D Bioprinting Project
5 | 9/24 | Synthetic Biology – Lab

In Class
- Synthetic Biology Lab
- check on K. hansenii cultures
- 2D Bioprinting Overview
- Presentation: example work
- Techniques
- direct drawing with various tools
- stamps
- stencils
- hand cut
- laser cut
- image to stencil Grasshopper template
- bioprinter
Homework
- check on SynBio plates
- begin planning and preparing for 2D Bioprinting Project
- gather tools
- create stencils
- prepare files
6 | 10/01 | Synthetic Biology Lab – Assessment and Troubleshooting

In Class
- Review of results from Synthetic Biology Lab
- document
- assessing results
- discussion
- troubleshooting
- recovering
- Demos
- making agar
- standard and black
- with and without antibiotics
- pouring agar plates
- making agar
- Review prepared materials/tools
Homework
- check on experimental plates
- observe and document growth as frequently as possible
- remove stencils as needed
- move plates to refrigerator as needed
- continue planning and preparing for 2D Bioprinting Project
- gather tools
- create stencils
- prepare files
- make agar plates
7 | 10/08 | Bioprinting Experimentation

In Class
- Lab work day
- Demos on manual workflows
- creating liquid cultures
- drawing with cells
- Demos on digital workflows
- Grasshopper for laser-cut stencils
- Grasshopper for bioprinter
- Demos on cell deposition methods for stencils
- manual application of cells by brush or spray
- ultrasonic vapor deposition
- Bioprinter demo
- Demo
- preserving plates
- others as needed
Homework
- check on plates
- observe and document growth as frequently as possible
- remove stencils as needed
- move plates to refrigerator as needed
- preserve as needed
- continue work on 2D Bioprinting Project
8 | 10/15 | Bioprinting Work Day

In Class
- Lab work day
- Demos on cell deposition methods for stencils
- manual application of cells by spray
ultrasonic vapor deposition
Bioprinter demo
Homework
- Work on bioprinting project
- observe and document growth as frequently as possible
- remove stencils as needed
- move plates to refrigerator as needed
- preserve as needed
9 | 10/22 | Bioprinting Work Day & Cellulose Harvest
In Class
- biorinting work day
- Harvest Bacterial Cellulose and begin washing protocol
- Lecture: Bacterial Cellulose in art and design
- Demo: plate preservation with resin
Homework
- complete bioprinting project
- document project
- complete washing protocol
- This process involves daily steps. Share the labor with classmates as needed to make this manageable
- research cellulose treatments
10 | 10/29 | Bioprinting Critique & Cellulose Conditioning

In Class
- Critique
- Bacterial cellulose treatment methods
Homework
- complete your bioprinting project documentation
- research bacterial cellulose treatment methods
- test bacterial cellulose treatment methods on sample pellicles
- plan bacterial cellulose project
11 | 11/05 | Finishing BC & Starting Mycelium

In Class
- Lecture: Bacterial cellulose in art and design
- review bacterial cellulose samples
- discuss project plans
- demo on project upload to website
- prepare substrate for mycelium inoculation
Homework
- finish bacterial cellulose project
- inoculate mycelium
- upload your bacterial cellulose project documentation using the provided template
12 | 11/12 | Bacterial Cellulose Critique

In Class
- bacterial cellulose critique
- presentation: mycelium in art, design, and manufacturing
- introduce Mycelium Project
- demo on vacuum forming molds
Homework
- prepare patterns for molds
- reading:
13 | 11/19 | MycoCasting

In Class
- create vacuum formed molds
- fill molds with inoculated substrate
Homework
- fill molds with inoculated substrate (if not done in class)
- document process
- reading: A Symbiotic View of Life: We Have Never Been Individual – paper by Scott Gilbert, et. al.
- related recommended readings:
- I Contain Multitudes – book by Ed Yong – Chapter 1 Living Islands
- Ways of Being – book by James Bridle – Chapter 1 Thinking Otherwise, Chapter 2 Wood Wide Web
- Staying with the Trouble – book by Donna Haraway
- related recommended readings:
14 | 11/26 | Thanksgiving
15 | 12/03 | Growing Networks
In Class
- check on mycelium castings
- demold castings if ready
- begin drying protocol
- discuss reading
Homework
- complete mycelium project
16 | 12/10 | Critique Mycelium Project
In Class
- 8:30-9:00 Install
- 9:00 Critique
- documentation
- course evaluations
Homework
- upload your mycelium project documentation using the provided template